Phthalates

Phthalates form a family of synthetic chemicals with a wide variety of uses, ranging from consumer to industrial products. There are different kind of phthalates and they are commonly used as softeners to make plastics, such as PVC, more flexible and durable.
Due to their wide use, phthalates can be found almost everywhere in our environment. All phthalates have not been thoroughly studied, but there is evidence that some of them are harmful to our health as they can, for example, interfere with our hormonal systems and cause allergies. As a result, the use of certain phthalates is already regulated both in Europe and globally.
Although phthalates are mostly used as plasticisers, they can also be found in, for example, adhesives, sealants, paints, rubber materials, wires and cables, flooring, packaging, food contact materials, medical devices and sports equipment.
The most known phthalates are ortho-phthalates,
such as bis(2-ethylhexyl) phthalate (DEHP)
and di-”isononyl” phthalate (DINP).
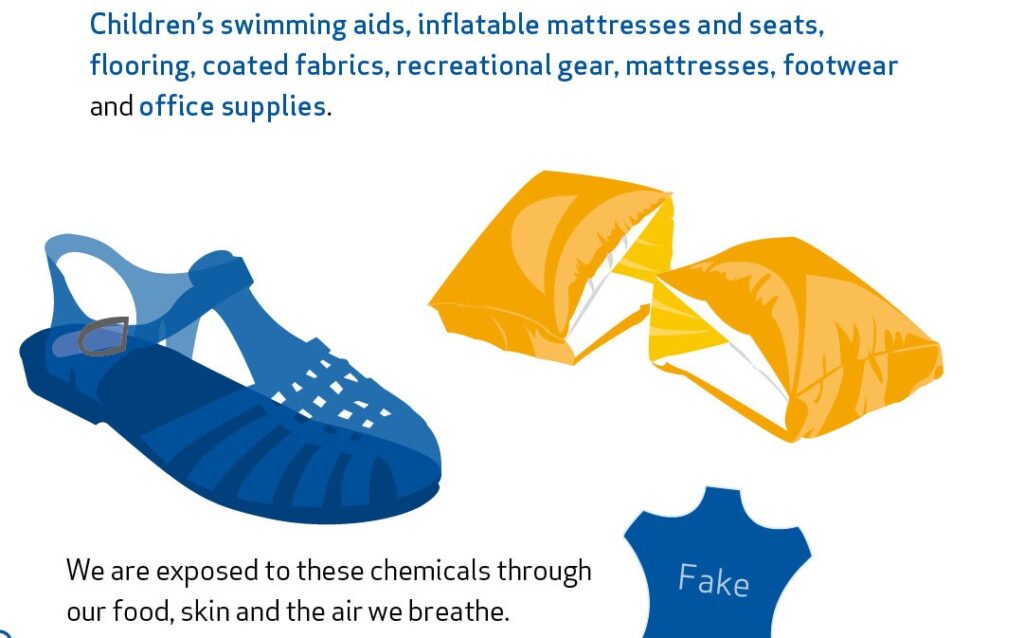
We are exposed to phthalates through food, skin, and air. As phthalates are not chemically bound in the materials they are added to, they can easily leach out or evaporate.
Several ortho-phthalates,
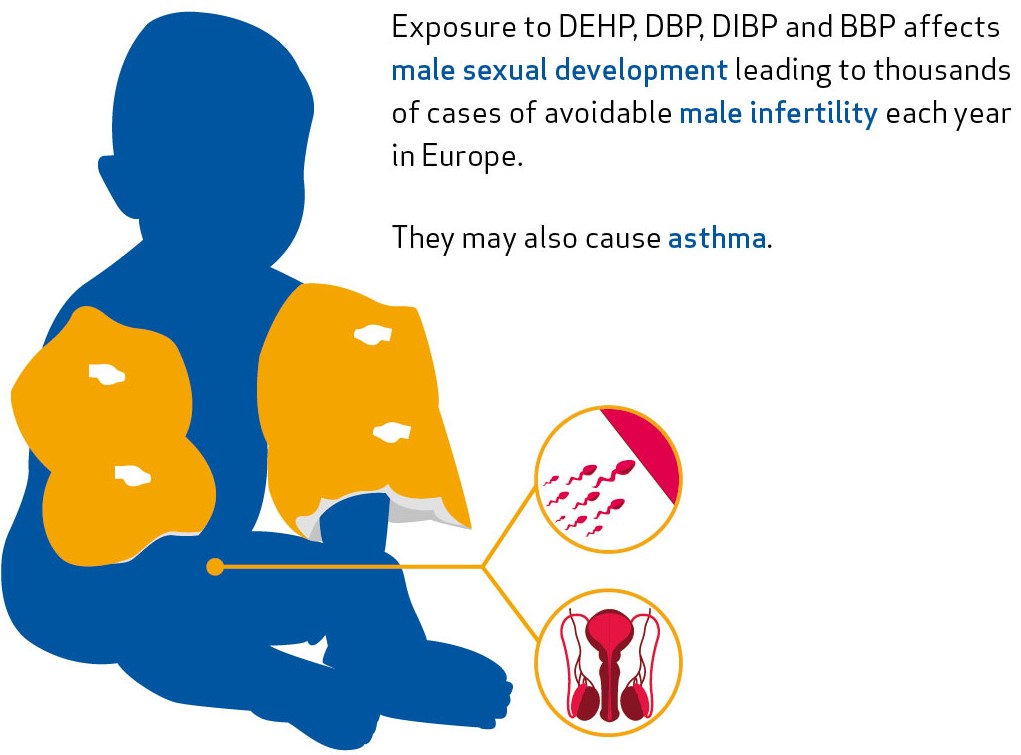
for example DEHP, dibutyl phthalate (DBP), diisobutyl phthalate (DIBP) and benzyl butyl phthalate (BBP)
may damage fertility or the unborn baby and interfere with our hormonal system. In particular, they affect the sexual development of boys which can lead to infertility in adults.
Apart from boys, pregnant women and young children in general have been found to be the most vulnerable groups to the effects of phthalates.
Some ortho-phthalates,
such as DBP, BBP, and DEHP
are also harmful to the environment.
Several ortho-phthalates, including DEHP, DBP, DIBP and BBP, are classified as toxic to reproduction (Repr. 1B). This means that they are presumed to have a negative effect on our ability to have children and on the unborn baby.
DBP and BBP are classified as very toxic to aquatic life (aquatic acute 1) and BBP also as very toxic to aquatic life with long lasting effects (aquatic chronic 1).
ECHA has assessed data on four groups of phthalates and phthalate-like substances:
- ortho-phthalates;
- isophthalates;
- terephthalates; and
- trimellitates.
Assessing structurally similar substances in groups speeds up identification of those substances which may need to be regulated. It also helps companies to avoid replacing hazardous chemicals by others with a similar concern.
Based on the assessment, the use of many ortho-phthalates may need to be limited in the future. Some will need harmonised classification and labelling or identification as substances of very high concern (SVHC). But there are also phthalates for which more data is needed to confirm the potential hazard, and for a few no regulatory actions are needed for the time being
The Candidate List of substances of very high concern (SVHC) contains several ortho-phthalates that are harmful for reproduction. Among those, five ortho-phthalates are identified as substances of very high concern also due to their endocrine disrupting properties – DIBP, DBP, BBP, DEHP, dicyclohexyl phthalate (DCHP) for human health, and DEHP and DCHP also for the environment.
Producers and importers of articles have to notify ECHA if their article contains a substance on the Candidate List within six months after the substance has been included in the list.
Altogether 14 phthalates are on the REACH Authorisation List. If a substance is placed on the Authorisation List, after a given date its use will be prohibited unless the European Commission authorises the company to continue its use. When applying for authorisation the company needs to demonstrate that the use of the substance is controlled, that there are no suitable alternatives and the benefits of continued use exceed the risks.
In November 2021, the Commission added endocrine disrupting properties to DEHP, DBP, BBP and DIBP, which were originally added to the list for being toxic to reproduction. The update of hazard properties to cover hormonal effects means that companies will need to apply for REACH authorisation for some uses that were previously exempted. These include, for example:
- uses of DEHP, for example in food contact materials, medical devices and immediate packaging of medicinal products (due to the hazards to the environment), and
- uses of BBP and DBP in immediate packaging of medicinal products.
Phthalates that are classified as toxic to reproduction (Repr. 1B) are restricted on their own and in mixtures that are intended for consumer use.
DEHP, DBP, DIBP and BBP are restricted in a wide range of products since July 2020. These include for example children’s swimming aids, flooring, coated fabrics and paper, recreational gear, mattresses, footwear, and office supplies. The restriction is expected to save about 2 000 boys each year from impaired fertility later in life.
Due to their EU-wide classification as toxic to reproduction, several phthalates are also restricted since November 2020 in consumer clothing or related accessories as well as in other textiles that come into contact with the skin. Phthalates covered by this restriction include: DEHP, DBP, DIBP, BBP, diisopentylphthalate 1,2-Benzenedicarboxylic acid, di-C6-8-branched alkyl esters, C7-rich, bis(2-methoxyethyl) phthalate, dipentyl phthalate, and dihexyl phthalate.
The restrictions also regulate the import of articles containing these phthalates.
Since 1999, the use of six phthalates, DINP, DEHP, DBP, di-“isodecyl” phthalate (DIDP), dioctyl phthalate (DNOP) and BBP, in children’s toys was prohibited under a temporary ban. In 2007, a permanent ban started to apply. This ban also broadened the scope to include childcare products that small children can put in their mouths. In January 2018, the restriction was further expanded to include DIBP.
Since July 2011, all phthalates classified as reprotoxic have generally been prohibited in toys and components of toys.
Old plastic items can still contain some of the phthalates that are no longer allowed to be used. This creates a challenge for circular economy as these phthalates first need to be detected and removed before the material can safely be recycled or reused.
Since 5 Jan 2021, the Waste Framework Directive requires manufacturers, importers and distributors of products containing substances of very high concern to notify about these products to ECHA’s SCIP database. Products containing phthalates that are on the REACH Candidate List must be notified.
Anyone can search the SCIP database to find products that contain certain phthalates. This is expected to help, for example, waste treatment operators in their daily work and will, in turn, contribute to circular economy. With more information available on hazardous chemicals, consumers can make more informed choices when buying products. Companies are also encouraged to use the information in SCIP database when considering how to replace such harmful substances.
The restriction of hazardous substances in the electrical and electronic equipment (RoHS) directive prohibits BBP, DBP, DEHP and DIBP in most electronic and electrical equipment since 22 July 2019. Products containing these chemicals are not allowed to be sold on the EU market. However, the restriction does not apply to products such as medical devices and control instruments that have been placed on the market before 22 July 2021.
The European Food Safety Authority (EFSA) assessed DBP, BBP, DEHP, DINP and DIDP in food contact materials in 2005 and again in 2019, considering new scientific evidence. The latest review introduced a tolerable daily intake level for the group of DBP, BBP, DEHP and DINP taking into account combined exposure to these phthalates. It was set on a temporary basis due to limitations and uncertainties identified in the assessment. The exposure to DBP, BBP, DEHP, DINP from food was found to be seven times below group safe level – while for DIDP the dietary exposure is 1 500 times below its safe level.
To follow up on the 2019 review, the European Commission has requested EFSA to conduct preparatory work ahead of the re-evaluation of the risks to public health from plasticisers such as phthalates used in food contact materials.
On October 27, 2017, the Commission issued a final phthalates rule (16 CFR part 1307) with an effective date of April 25, 2018. The final phthalates rule makes permanent the interim prohibition on children’s toys that can be placed in a child’s mouth and child care articles that contain concentrations of more than 0.1 percent of DINP. The phthalates rule extends this prohibition to cover all children’s toys and child care articles containing concentrations of more than 0.1 percent of DINP. The phthalates rule also lifts the interim prohibitions on children’s toys that can be placed in a child’s mouth and child care articles that contain concentrations of more than 0.1 percent of DNOP or DIDP. In addition, the phthalates rule prohibits children’s toys and child care articles that contain concentrations of more than 0.1 percent of diisobutyl phthalate (DIBP), Di-n-pentyl phthalate (DPENP), di-n-hexyl phthalate (DHEXP), and dicyclohexyl phthalate (DCHP).
The CPSIA’s permanent prohibition concerning DEHP, DBP, and BBP remains in effect. Thus, any children’s toy or child care article that contains concentrations of more than 0.1 percent of the following phthalates is prohibited:
- di-(2-ethylhexyl) phthalate (DEHP),
- dibutyl phthalate (DBP),
- benzyl butyl phthalate (BBP),
- diisononyl phthalate (DINP),
- diisobutyl phthalate (DIBP),
- di-n-pentyl phthalate (DPENP),
- di-n-hexyl phthalate (DHEXP), and
- dicyclohexyl phthalate (DCHP).
The definitions of “children’s toys” and “child care articles” are discussed below. Please read the discussion below concerning component parts and inaccessibility to understand how the prohibition may apply to your product.
- The manufacture, sale, or distribution of children’s toys and child care articles containing BBP, DBP, or DEHP at levels greater than 0.1%.
- The manufacture, sale, or distribution of toys and child care articles intended for the use of a child under 3 years old, if that product can be placed in the mouth, and it contains DIDP or DINP at levels greater than 0.1%.